Paul M. Zoll, M.D.
The Early Research
1952
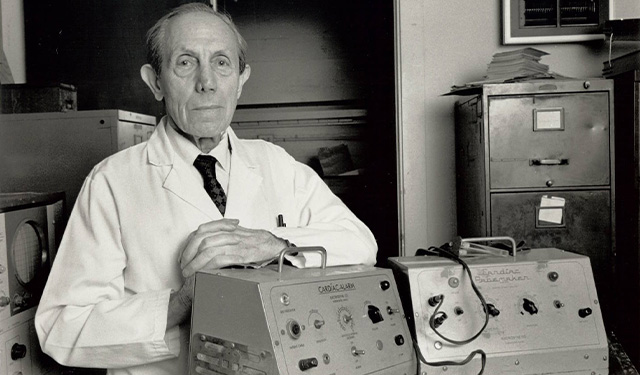
While Chief of the Cardiac Clinic at Beth Israel Hospital, Paul M. Zoll, M.D., demonstrates that external electrical stimulation of a patient's chest during cardiac arrest can produce an effective heartbeat.
1956
Dr. Zoll is the first physician to successfully use external defibrillation to regulate heart rhythms in patients. This discovery contributes significantly to the decrease in heart disease mortality.
With technical collaborators, Dr. Zoll develops a way to display the heart's cardiac electrical activity on an oscilloscopic screen. This leads to the development of cardiac monitors and other programs found in modern cardiac care units.
1960
Dr. Zoll discovers that external electrical countershock is effective in terminating supraventricular tachycardia and ventricular tachycardia. This procedure becomes widely used in the treatment of these arrhythmias and is found to be safer than administering large doses of antiarrhythmic drugs.

1964
Dr. Zoll develops a method for long-term direct electrical heart stimulation through an implanted pacemaker.

1973
Dr. Zoll receives the Albert Lasker Award for Clinical Medical Research, the most coveted award in medical science.
Photo courtesy of laskerfoundation.org
1977
Dr. Zoll becomes Clinical Professor of Medicine, Emeritus, at Harvard Medical School.
ZOLL Medical Corporation
1980
ZOLL® is incorporated by the company's co-founders, including Dr. Zoll, Leigh Stein, and Thomas Claflin. William Bright, Chairman, and Rolf Stutz, CEO, subsequently join ZOLL.
1983
The ZOLL NTP® 1000, a non-invasive temporary pacemaker based on Dr. Zoll's research, is introduced.
1988
Launches the ZOLL PD 1200™ pacemaker/defibrillator/monitor, the first to combine proven pacing and defibrillating technology in a reliable, easy-to-use, compact unit.
1989
Introduces adult multi-function electrodes, which provide well-tolerated pacing, effective defibrillation, cardioversion, and monitoring.
1992
ZOLL becomes a public company.
Releases the PD 1400 pacemaker/defibrillator/monitor, the smallest device of its kind available for critical patient transport and pre-hospital treatment.
The American Heart Association elevates non-invasive pacing to the initial treatment of choice for certain serious patient conditions. ZOLL is the market leader for this technology.
1994
Receives 510(k) marketing clearance from the FDA for the PD/D 2000 automated external defibrillator (AED)/advisory defibrillator.
1995
Establishes a subsidiary in the Netherlands.
Launches ZOLL 1600, the first fully upgradeable and configurable AED/manual external defibrillator.
Introduces the first modular AC power supply/battery charger for use in hospitals on the ZOLL 1400, 1600, and 2000 family of defibrillators.
1996
Acquires Westech Mobile Solutions, adding software-based information products to the pre-hospital product line.
Makes an equity investment in LIFECOR, Inc., a medical equipment company that makes LifeVest®, the first and only wearable defibrillator.
Introduces the ZOLL 1700, the first fully integrated AED/manual external defibrillator for basic life support (BLS) and advanced cardiac life support (ACLS) in a hospital.
Announces the new Base PowerCharger™ 4 x 4, the first system in the industry to offer a practical solution for improving the reliability, effectiveness, and cost of battery management.
1997
Launches RescueNet®, the only complete EMS information management system that helps improve patient care, operational efficiency, and agency compliance.
Unveils a tablet computer-based 12-lead system to help identify the onset of cardiac arrest.
1998
Introduces the M Series® monitor/defibrillator, the most advanced defibrillator worldwide. The product is approximately one-third the size and half the weight of any competitive full-featured defibrillator.
1999
Establishes subsidiaries in Canada and Germany.
Debuts the Rectilinear Biphasic™ defibrillation waveform and is given FDA clearance to label this technology as clinically superior to monophasic defibrillators for the conversion of ventricular fibrillation in high-impedance patients and for cardioversion of atrial fibrillation.
Acquires Pinpoint Technologies to provide a complete information management solution to the EMS market.
Introduces Smart Batteries, an industry first providing users with an accurate run time display.
2001
ZOLL establishes subsidiaries in Australia and France.
Launches the M Series CCT for critical care transport.
The ZOLL AED Plus® defibrillator is designed in cooperation with hundreds of first responders and EMS agencies.
2002
Receives 510(k) marketing clearance from the FDA for its AED Plus defibrillator and the new CPR-D-padz® electrodes, the first system to provide instantaneous feedback on the depth and rate of chest compressions during CPR.
ZOLL provides an initial grant to help establish the AED Instructor Foundation.
ZOLL and LIFECOR, Inc. announce an agreement to sell LIFECOR's LifeVest wearable defibrillator to hospitals for patient use in the United States and Canada.
The M Series CCT is selected as the defibrillator of choice for the U.S. Military Patient Movement Item Program. The agreement, worth $8.9 million, is the single largest order in ZOLL's history.
2003
Announces a relationship with Advanced Circulatory Systems, Inc. to bring the ResQPOD® circulatory enhancer to market.
Enters an agreement with Revivant Corporation to commercialize the AutoPulse® non-invasive cardiac support pump, a new FDA-approved portable device that automates chest compressions and increases blood flow to the brain and heart more consistently than manual CPR.
2004
Establishes a subsidiary in Austria.
Renames its Pinpoint Technologies subsidiary ZOLL Data Systems to better reflect the EMS market.Acquires Infusion Dynamics, Inc., which manufactures a unique fluid resuscitation product called the Power Infuser®.
Pediatric capability for the AED Plus receives clearance from the FDA.
2005
Introduces the ZOLL AED Pro® for professional rescuers.
Launches the E Series® monitor/defibrillator, designed to meet the specific demands and extreme conditions of the EMS environment.
2006
Completes the acquisition of the assets of LIFECOR, Inc.
Launches the R Series® monitor/defibrillator, the first and only Code-Ready® defibrillator for hospitals.
2007
Receives market clearance for E Series with Real CPR Help® technology.
Purchases the temperature management assets of Radiant Corporation.
Surpasses $300 million in revenues.
Receives clearance to market E Series and AED Pro with See-Thru CPR® technology whose functionality helps minimize interruptions in CPR.
2008
STEMI solutions offer enhanced transmission technology for 12-lead ECG.
AED Pro is approved for use on MEDEVAC helicopters, receiving certification that it meets the US Defense Department’s criteria for medical devices after extensive testing by the US Army’s Aeromedical Research Laboratory.
AutoPulse approved for use in China.
LifeVest wearable defibrillator exceeds 10,000 prescriptions.
ZOLL and Welch Allyn enter strategic alliance; ZOLL becomes exclusive distributor of Propaq® LT.
Reaches $398 million in revenues, an increase of 29% over the previous year.
2009
Purchases intravascular temperature management (IVTM™) assets from Alsius Corporation.
New R Series BLS and R Series Plus monitor/defibrillators receive FDA clearance.
2010
Propaq MD monitor/defibrillator and Propaq M monitor granted clearance by the FDA.
LifeVest wearable defibrillator physician prescriptions top 30,000.
2011
AutoPulse Circulation Improving Resuscitation Care (CIRC) trial concludes successfully; first large-scale resuscitation trial to reach a statistically significant result.
Receives Frost & Sullivan 2010 Market Leadership Award for demonstrated excellence in capturing the highest market share of the North American external defibrillator market.
PocketCPR® for iPhone – a training app – is the first to support new 2010 AHA/ERC/ILCOR Guidelines.
Reports record Q4 and annual revenues; breaks the $500 million mark with fiscal 2011 revenues of $523.7 million.

Launches iPad app for ePCR; EMS teams can now use iPads to complete electronic patient care reports.
2012
Asahi Kasei acquires ZOLL for $2.21 billion.
Introduces the first pediatric electrodes that report CPR quality on children up to eight years old.
Establishes new Japanese subsidiary Asahi Kasei ZOLL Medical (AZM).
X Series® monitor/defibrillator receives 510(k) clearance from the FDA.
2013
First medical device company to sign pledge to make real-time patient information available to clinicians, such as from EMS services to hospitals, in order to reduce preventable deaths.
Introduces the Fully Automatic AED Plus with Real CPR Help, automatically delivering a shock when it detects a shockable heart rhythm.
2014
Expands product portfolio to include ventilation with the acquisition of assets of Impact Instrumentation, Inc.
Acquires Philips InnerCool® temperature management business.
2015
Acquires Advanced Circulatory Systems, Inc., of Roseville, Minn., developer of the ResQPOD.
ResQCPR® system receives premarket approval from the FDA as it is shown to profoundly increase blood flow to the heart and other vital organs in pre-clinical studies.
2016
Receives market approval for intravascular temperature management technology to treat sudden cardiac arrest in Japan.
ZOLL AED 3® and the ZOLL AED 3 BLS automated external defibrillators are approved for marketing and distribution in Europe.
Establishment of the ZOLL EMT Scholarship, awarded to eleven caregivers in the program's inaugural year.
2017
Health Canada approves the ZOLL AED 3 and the ZOLL AED 3 BLS automated external defibrillators for marketing and distribution in Canada.
ZOLL Hospital Wearable Defibrillator (HWD) receives premarket approval from the FDA.
2018
Propaq M selected by U.S. Air Force and U.S. Army as their deployable vital signs monitor.
ZOLL is the first company to receive premarket approval from the FDA on its full portfolio of defibrillators.
VEST trial shows LifeVest wearable defibrillator reduces total mortality by 36% at 90 days.
Heroes for Life program launched to honor sudden cardiac arrest survivors and the "unexpected heroes" who helped save them with a ZOLL AED.
Respond™ app introduced to improve communication and navigation among EMS dispatchers, crews, and clinical staff.

Z Vent® transport ventilator introduced for pre- and intra-hospital use.
2019
Acquires Payor Logic, Inc., a company that specializes in best-in-class accounts receivable (A/R) software solutions for the healthcare industry.
Acquires Golden Hour Data Systems, Inc. Accelerates patient charting and revenue cycle management to the EMS market.
Introduces µCor™ (pronounced "Micro Core") Heart Failure and Arrhythmia Management System (HFAMS), new technology to improve the management of acute heart failure patients.
Acquires TherOx®, Inc., which offers SuperSaturated Oxygen (SSO2) Therapy, a new FDA-approved treatment to help the most severe heart attack patients.
Acquires Mobilize Rescue Systems™, which offers interactive trauma systems to help bystanders save lives during medical emergencies.

Rebrands Payor Logic solution as ZOLL AR Boost™ to better reflect the potential impact of this technology on a wider segment of the healthcare market.
ZOLL completes acquisition of Cardiac Science.
2020
FDA approves next-generation TherOx system for left anterior descending ST-elevation myocardial infarction (LAD STEMI) heart attack patients.
2021
Acquires Respicardia, Inc., a provider of novel implantable neurostimulators for the treatment of moderate to severe Central Sleep Apnea (CSA).
FDA approves next-generation remedē® EL-X System to treat moderate to severe CSA.
Acquires Itamar Medical, a device and digital health company that provides at-home testing for sleep apnea.
2022
ZOLL launches the Arrhythmia Management System for more informed treatment plans adding biometric data to traditional mobile cardiac telemetry information.
LifeVest has been worn by more than 1 million patients.
2023
ZOLL remedē System receives FDA approval for conditional use with MRI, giving patients access to a wider range of imaging options.
ZOLL introduces Autopulse® NXT, a significant advance in mechanical CPR technology. The device is designed to provide continuous circumferential compressions on a wider range of patient sizes while also providing unhindered visibility of the coronary arteries under fluoroscopy in the cath lab.